We wonder just exactly what this vaccine was, considering the Pfizer bivalent injection was only approved in New Zealand on 21 December 2022, has only been available since March 2023 and as a special April Fool’s joke has been more widely available to those over 30 from 1 April 2023... NZDSOS
1st Multivalent Vaccine Death in NZ
Some of our supporters have been studying the US VAERS (Vaccine Adverse Events Reporting System) database, connecting it with NZ information.
It appears that the more serious and unexpected adverse events reported to CARM, also get reported to the VAERS system.
A report (copied below) was discovered and has piqued our interest. It appears a 70 year old New Zealand woman received her fourth covid injection on 30 October 2022. As reported by her daughter, within an hour of receiving the vaccine she went into a coma and then died 4 days later.
Her AEFI-A (adverse event following immunisation) number from the NZ regulatory authority (Medsafe) is AEFI-A-079545.
Events in such close proximity to a vaccine are disturbing in themselves but also of concern is that, according to the VAERS report, she received a Pfizer COMIRNATY (= BNT162b2) MULTI-VALENT NOS (not otherwise specified) vaccine.
We wonder just exactly what this vaccine was, considering the Pfizer bivalent injection was only approved in New Zealand on 21 December 2022, has only been available since March 2023 and as a special April Fool’s joke has been more widely available to those over 30 from 1 April 2023.
What did she receive? What multivalent covid vaccines were available in NZ in October 2022? Was it a trial injection?
We note that Kiwis have been involved in several clinical trials with covid vaccines.
A trial studying the use of the Pfizer vaccine administered concomitantly with the flu vaccine has had trial participants in NZ but the study was completed on 5 Oct 2022 prior to this New Zealand woman receiving her dose of vaccine.
Another study sponsored by Novavax is being conducted and has participants in New Zealand. It is investigating the effectiveness of a single combined vaccine to prevent both influenza and covid 19. This study appears to have started in December 2022 though, so after this woman received her multivalent vaccine and died.
Kiwi Vax developed by Vaccine Alliance Aotearoa New Zealand has shown promise in pre-clinical trials but is yet to be given to human volunteers to test safety and effectiveness.
Perhaps there are other trials under way.
This adverse reaction/death would not have been included in the last Medsafe Safety Report covering the period up to the end of November 2022 and published on 14 Dec 2022. Medsafe have no further adverse event reports scheduled over three months later. Given the rollout of the bivalent vaccine on 1 March 2023, this is unacceptable.
If you have any information about what this New Zealand woman may have received, we’d be interested to know.
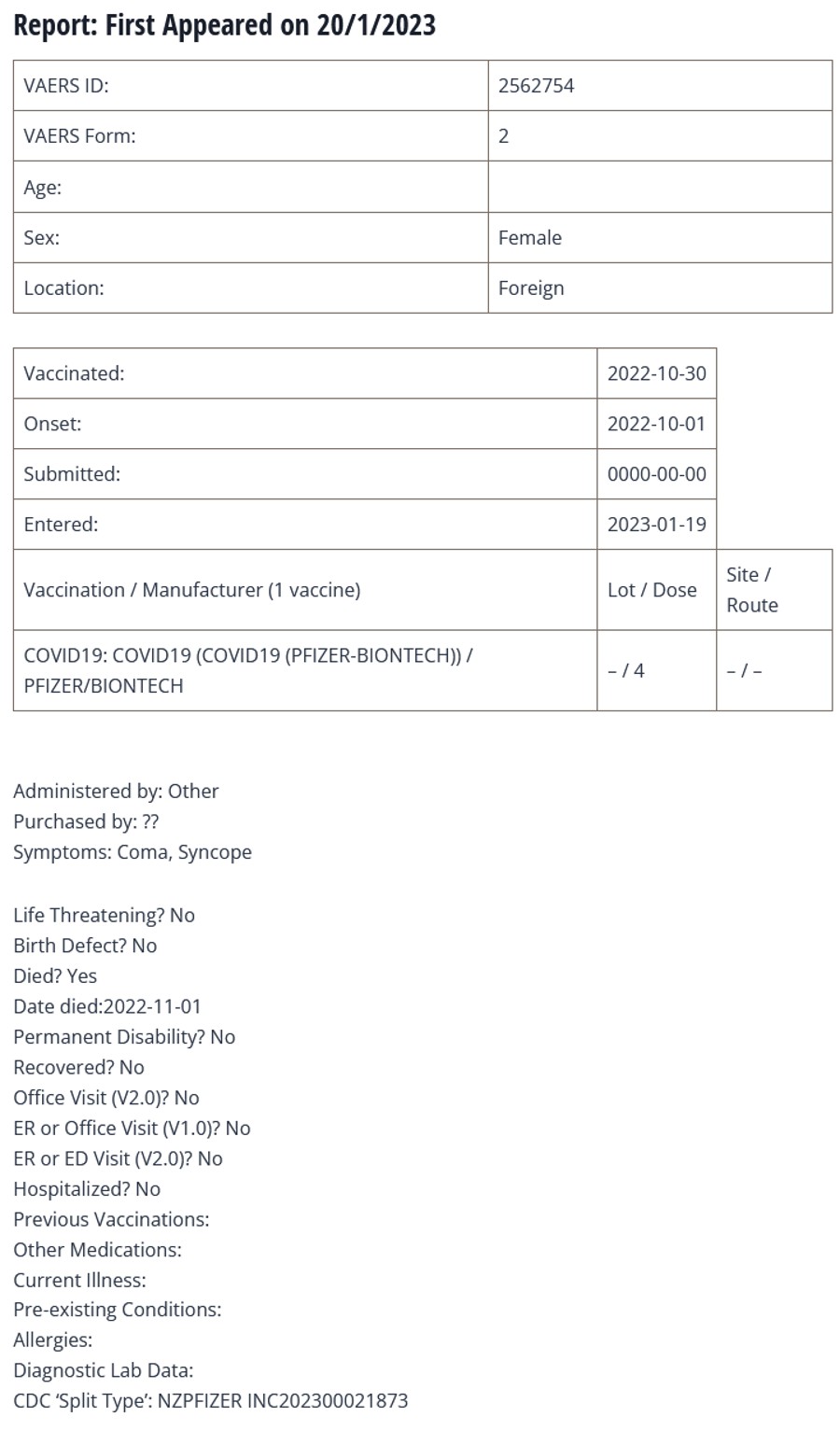
Write-up: Coma; Syncope; This is a spontaneous report received from a contactable reporter(s) (Consumer or other non HCP) from Regulatory Authority. Regulatory number: AEFI-A-079545. A 70-year-old female patient received BNT162b2 multivalent nos (COMIRNATY MULTIVALENT NOS), on 30 Oct 2022 as dose 4 (booster), single (Batch/Lot number: unknown) for covid-19 immunisation.
The patient’s relevant medical history and concomitant medications were not reported. Vaccination history included: Covid-19 vaccine (Dose 3; Manufacturer Unknown), for COVID-19 immunisation; Covid-19 vaccine (Dose 2; Manufacturer Unknown), for COVID-19 immunisation; Covid-19 vaccine (Dose 1; Manufacturer Unknown), for COVID-19 immunisation.
The following information was reported: COMA (death, medically significant) with onset Oct 2022, outcome “fatal”; SYNCOPE (death, medically significant) with onset Oct 2022, outcome “fatal”.
The patient date of death was Nov 2022. Reported cause of death: “Coma”, “Syncope”. Clinical course: Reporters description of AEFI included within an hour of being given the Covid booster reporter” mum went into a coma. Died 4x days later. Reportedly, died-unrelated to Med (presented as reported).
The reporter considered “coma” and “syncope” not related to BNT162b2 multivalent nos. The information on the batch/lot number for BNT162b2 multivalent nos has been requested and will be submitted if and when received.; Reported Cause(s) of Death: Coma; Syncope
Photo: nzdsos.com